Bromothymol Blue Turns Yellow in the Presence of
Bromothymol blue is referred to as a dye that is used as an indicator in determining pH. It is basically used in applications that require measuring substances that would have a relatively neutral pH. A very common use is for measuring the presence of carbonic acid in a liquid. It is typically sold in solid form as the sodium salt of the acid indicator. Let's learn bromothymol blue in detail and discuss some important questions.
Also read: Importance of pH in Everyday Life
Key takeaways: Bromothymol blue, bromothymol sulfonephthalein, pH indicator, organic chemistry.
What is Bromothymol Blue?
[Click Here for Sample Questions]
Bromothymol blue or bromothymol sulfonephthalein is used as a pH indicator and is in low concentration. It is an indicator of pH levels ranging from 6.0 to 7.6. In common use, it is used to measure carbonic acid in liquid and measure toxicity. The presence of bromothymol blue causes the liquid to change from yellow to blue-green. It contains an antimycotic agent, cycloheximide, antimicrobial agents, chlortetracycline, and chloramphenicol. It is found in solid form as it contains sodium salt and then gets converted for use.

T The other names of bromothymol blue are Di Bromothymol Sulfo Phthalein, Dibromothy molsulfon phthalein, and 3,3'.
| C27H28Br2O5S | Bromothymol Blue |
| Density | 1.25 g/cm3 |
| Molecular Weight/ Molar Mass | 624.384 g/mol |
| Boiling Point | 614.26° C at 760 mmHg |
| Melting Point | 202 °C |
| Chemical Formula | C27H28Br2O5S |
Also Read: Coordination Compounds
Bromothymol Blue Formula
[Click Here for Sample Questions]
The chemical formula of bromothymol blue is C27H28Br2O5S. BTB is a heavy-weight molecule of 625 g/mol. It has a unique structure of three aromatic benzene rings. The first ring is connected with the sulfur atom and has a thym group that has two oxygen atoms bonded by a double bond. Another oxygen atom is attached to the sulfur atom via a single bond. Both the second and third benzene rings have a bromine atom, a methyl group, a tert-butyl group, and an alcohol group attached. In another way, bromothymol blue is a member of 2,1-benzoxazoles which is 2,1-benzoxathiole 1,1-dioxide in which both of the hydrogens at position 3 have been substituted by 3-bromo-4-hydroxy-5-isopropyl-2-methylphenyl groups.
Also Read: Carbon and its Compounds
Bromothymol Blue Structure and Properties
[Click Here for Sample Questions]
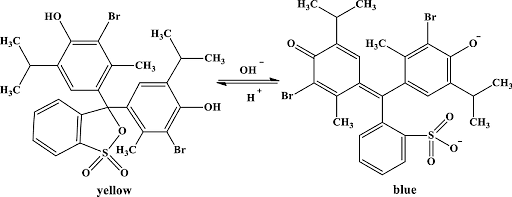
Bromothymol Blue is a type of dye used as an indicator of pH level. It is a weak acid and can act as an acid or in base form based on the pH level of the solution. Its color changes depending on the solutions. It turns yellow if it is an acidic solution, blue in basic solutions, and green if it is a neutral solution.
Bromothymol Blue structure:
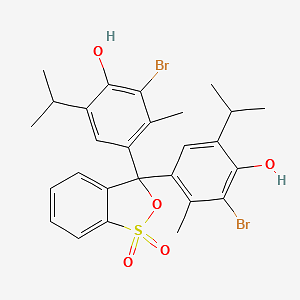
The chemical formula is C27H28Br2O5S. A member of the class of 2,1-benzoxazoles that is 2,1-benzoxathiole 1,1-dioxide in which both of the hydrogens at position 3 have been substituted by 3-Bromo-4-hydroxy-5-isopropyl-2-methyl phenyl groups. Its protonated form has an absorption at 927 nm that makes it turn into yellow color. Its deprotonated form has an absorption point at 602 nm that turns it into blue light when in basic solution. And it trunks blue when put in neutral solutions.
The below table indicates the physical properties of bromothymol blue:
| Odor | Odorless |
| Appearance | Yellow in acid solution, Blue in basic solutions, and green in neutral solution. |
| Covalently-Bonded Unit | 1 |
| Complexity | 818 |
| pH | 7.6 |
| Solubility | Sparingly soluble or solubility level is low in the water |
Synthesis and Preparation of Bromothymol blue
[Click Here for Sample Questions]
Reagents required for this process:
- 0.1 grams of bromothymol blue powder
- 16mL of 0.01 N sodium hydroxide
- Distilled Water
Steps followed in the process:
- Dissolve the given bromothymol blue powder in sodium hydroxide solution
- Dilute the solution after the above process with water not more than 250 mL.
Also Read: Isomerism
Uses of Bromothymol Blue(C27H28Br2O5S)
[Click Here for Sample Questions]
Bromothymol Blue is used for various purposes including :
- Bromothymol blue can be used as an indicator of CO2 level and to observe photosynthetic activities.
- It is a common demonstration of the pH indicator properties and measures the pH level of any solution.
- Bromothymol blue indicator is used in conjunction with phenol red in examining the fungal asparaginase enzyme activity when it turns pink.
- Bromothymol blue turns blue that indicating an increase in pH which determines the enzyme activity. However, according to a recent study, the red part is useful to determine activity because of the bright yellow ring form in the enzyme activity zone.
- Bromothymol is also used to detect premature membrane rupture. So bromothymol turns blue after in contact with leaking fluid amnion.
- It is also used in the laboratory as a stain on a biological slide.
Things to Remember
- Bromothymol blue is mainly used to measure the pH level of any liquid.
- The chemical formula of bromothymol blue is C27H28Br2O5S.
- It turns Yellow in acid solution, Blue in basic solutions, and green in neutral solution.
- Its pH level ranges from 6.0 to 7.6.
- Bromothymol blue has various usages in finding results about various solutions.
Also Read: Charles Law Formula
Sample Questions
Ques. What bromothymol blue indicates the presence of any substances? (2 marks)
Ans. Bromothymol blue (BMB) is an indicator dye that turns yellow when in contact with acid. When CO2 is added to the solution, carbonic acid is produced which lowers the solution's pH.
Ques. How toxic is bromothymol blue? (2 marks)
Ans. It causes Chronic effects on humans. It can cause damage to human organs lungs and mucous membranes. It may have adverse effects on humans and is very dangerous for ingestion. skin touch (irritant), and inhalation.
Ques. What is the color of bromothymol blue in a base? Explain. (3 marks)
Ans. Bromothymol blue is an acid indicator and based on the pH level of the solution, it indicates whether it is an acid or base form. It changes to purple or blue color in acid solution and green in neutral solution. However, bromothymol blue is a weak acid but it can stay in both acid and base form. To conclude, bromothymol blue turns yellow in acidic solution, blue in basic solution, and green in neutral solution.
Ques. How harmful is the consumption of Bromothymol blue? (3 marks)
Ans. The consumption of bromothymol blue can cause irritation to the eyes and skin and can cause toxicity if swallowed. However tiny consumption can cause digestion malfunction and causes nausea and vomiting, and even death if it continues for long. Drinking water will treat incidental swallowing.
Ques. What is the color of bromothymol blue in neutral solution? Why? (3 marks)
Ans.Bromothymol blue is green in color when put in a neutral solution. It is a heavy acid and in neutral water, it is bluish-green. The deprotonation of the results in neutral form is a type of structure that is highly conjugated and because of this, the difference in colors occurs. The greenish color of the neutral solution is responsible for an intermediate of the deprotonation process.
Ques. What is the chemical formula of bromothymol blue? Explain. (3 marks)
Ans. The chemical formula of bromothymol blue is C27H28Br2O5S. Bromothymol blue is a member of the class of 2,1-benzoxathioles that is 2,1-benzoxathiole 1,1-dioxide in which both of the hydrogens at position 3 have been substituted by 3-Bromo-4-hydroxy-5-isopropyl-2-methyl phenyl groups. It has a role as an acid-base indicator, a dye, and a two-color indicator.
Ques. Bromothymol blue added to a solution imparts blue color, what is the pH of this solution? (1 mark)
Ans.If the solution's pH level is 5 then it is acidic in nature and if the solution's pH level is 9 then it has a base form.
Ques. Is there a benefit to using different pH indicators for carbon utilization tests? (3 marks)
Ans. Choice of pH indicator depends upon the factor, that different indicators provide changes as per to their limits. For example, if you are using Bromocresol Purple, you will see it purple at neutral pH and turns yellow near pH 5. Now the question is if your bacterial stain does not produce enough acid to go near that pH, this indicator will not work, and even though your bacterium is creating acid from carbon and the result can be seen. To see the result, you have to use phenol red (6.8 to 8.4; where minor change is examined).
Ques. What color change occurs when bromothymol blue is put into acidic condition? (3 marks)
(a) Yellow
(b) Blue
(c) Green
(d) Red
Ans. The correct option is yellow. Bromthymol Blue is a type of dye used as an indicator to determine pH. Bromthymol blue is a weak acid. It can be either acid or base form, depending on the pH of the solution. This reagent is yellow in acidic solutions, blue in basic solutions, and green in neutral solutions.
Ques. Why is alcohol added in the preparation of bromothymol blue solutions? (2 marks)
(a) To help the bromothymol blue dissolve
(b) To neutralize the solution
(c) To create a clear colored solution
(d) It makes the bromothymol blue to react in the presence of basic conditions
Ans. The alcohol can be used to make it easier to dissolve the bromothymol in water since bromothymol is highly soluble in alcohol but dissolves with difficulty in the water.
Also Read:
Also Read:
Source: https://collegedunia.com/exams/bromothymol-blue-structure-molecular-mass-properties-and-uses-chemistry-articleid-6448
Post a Comment for "Bromothymol Blue Turns Yellow in the Presence of"